Pfizer Inc. announced a recall of a popular birth control.
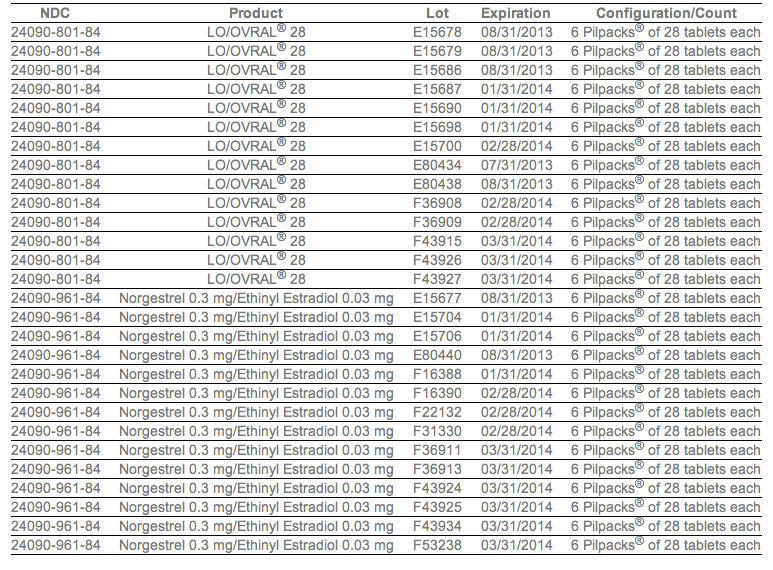
The company said Tuesday that it would be recalling Lo/Ovral-28 (norgestrel and ethinyl estradiol) tablets and 14 lots of Norgestrel and Ethinyl Estradiol Tablets (generic). The recall follows an investigation that found some blister packs may contain an inexact count of inert or active ingredient tablets and that they may be out of sequence.
“As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy,” Pfizer said in a statement.
The products are packaged in blister packs which hold 21 tablets of active ingredients and seven tablets of inert ingredients.
The company says that packaging defects don’t pose any immediate health risks but advises users exposed to affected packaging to begin using a non-hormonal form of contraception immediately and notify their doctor.
Pfizer also advised users to return the product to the pharmacy.
“Any adverse events that may be related to the use of these products should be reported to Akrimax Medical Information at 1-877-509-3935 (8 AM to 7 PM Mon-Fri CST) or to FDA’s Med Watch Program either online, by regular mail or by fax,” noted Pfizer.
In a press release, Pfizer provided the lot numbers of affected packs: